MoNo – the Company
 MoNo chem-pharm Produkte GmbH was founded in 2000 as a manufacturing company and is affiliated with the long-standing business Sigmapharm Arzneimittel GmbH, which has been serving the health sector for more than seventy years. Decades of expertise, state-of-the-art production facilities and contract manufacturing at the highest level: MoNo chem-pharm Produkte GmbH sees itself as an all-round service provider and manufacturer. More about the company
MoNo chem-pharm Produkte GmbH was founded in 2000 as a manufacturing company and is affiliated with the long-standing business Sigmapharm Arzneimittel GmbH, which has been serving the health sector for more than seventy years. Decades of expertise, state-of-the-art production facilities and contract manufacturing at the highest level: MoNo chem-pharm Produkte GmbH sees itself as an all-round service provider and manufacturer. More about the company
Manufacturing
Mono chem-pharm Produkte GmbH specializes in the development and production of all types of pharmaceutical liquids (sterile and non-sterile, aqueous, oily, alcoholic, viscous). Our state-of-the-art facilities for the aseptic production of sterile liquid products are the lifeblood of our business. All manufacturing processes are strictly controlled and monitored in accordance with current GMP guidelines and ISO standards, enabling us to guarantee the highest quality. As a team, we at MoNo offer a comprehensive range of services for product development, clinical trial supplies, process validation and analysis. Our partnership with Sigmapharm Arzneimittel GmbH expands our range of services with regard to the compilation of submission documentation, the development of packaging design solutions as well as warehousing and dispatch logistics.
Sterile Solutions
STERILE SOLUTIONS NON-STERILE SOLUTIONS SMALL BATCH SERVICE STUDY MEDICATION SERIALISATION REPACKAGING Our production sites in…
Non-Sterile Solutions
STERILE SOLUTIONS NON-STERILE SOLUTIONS SMALL BATCH SERVICE STUDY MEDICATION SERIALISATION REPACKAGING MoNo chem-pharm Produkte GmbH…
Small Batch Service
STERILE SOLUTIONS NON-STERILE SOLUTIONS SMALL BATCH SERVICE STUDY MEDICATION SERIALISATION REPACKAGING Pharmaceuticals or medical devices…
Study Medication
STERILE SOLUTIONS NON-STERILE SOLUTIONS SMALL BATCH SERVICE STUDY MEDICATION SERIALISATION REPACKAGING Compliance with GMP regulations…
Serialisation
STERILE SOLUTIONS NON-STERILE SOLUTIONS SMALL BATCH SERVICE STUDY MEDICATION SERIALISATION REPACKAGING In early 2019, the…
Repackaging
STERILE SOLUTIONS NON-STERILE SOLUTIONS SMALL BATCH SERVICE STUDY MEDICATION SERIALISATION REPACKAGING Repackaging Should legislation and…
Product Development
 In addition to the contract manufacturing of sterile and non-sterile liquid products, our services also span the entire spectrum of product development services in this product segment. Our extensive expertise enables us to support you and collaboratively develop your new product from concept to manufacture to market launch. More about Product Development
In addition to the contract manufacturing of sterile and non-sterile liquid products, our services also span the entire spectrum of product development services in this product segment. Our extensive expertise enables us to support you and collaboratively develop your new product from concept to manufacture to market launch. More about Product Development
Quality Control
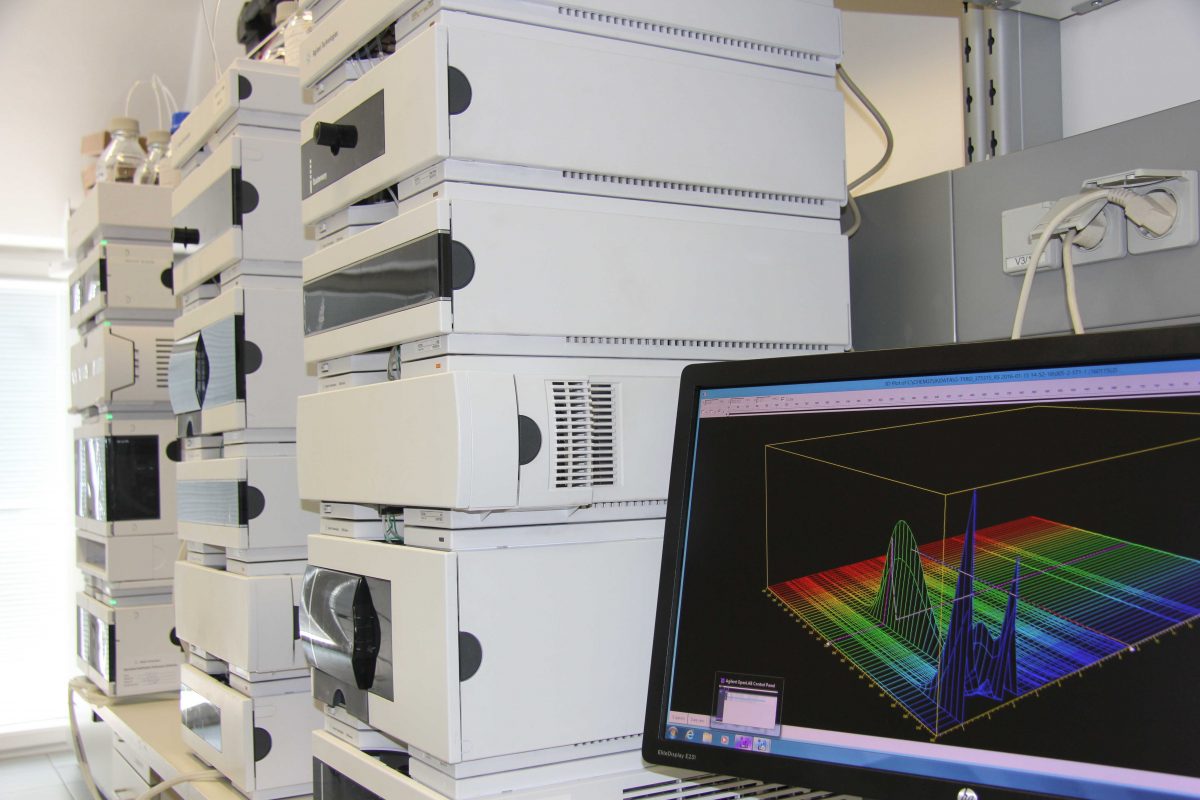 Our highly qualified and experienced quality control team performs state-of-the-art chemical and microbiological analyses. In accordance with regulations, we check everything from the raw materials to the packaging materials to the finished product and respond flexibly to your individual needs. More about Quality Control
Our highly qualified and experienced quality control team performs state-of-the-art chemical and microbiological analyses. In accordance with regulations, we check everything from the raw materials to the packaging materials to the finished product and respond flexibly to your individual needs. More about Quality Control
©2019 Mono.co.at | privacy statement | disclaimer | imprint